Properties of acids酸的性质
1. They are liquids.
它们是液体。
2. They are solutions of compounds in water.
它们是化合物在水中的溶液。
3. If concentrated they can be corrosive.
如果浓缩,它们可能具有腐蚀性。
4. Acids taste sour (for example, vinegar).
酸味酸(例如,醋)。
5. Turn blue litmus paper red - this is an easy test for an acid!
将蓝色石蕊试纸变红 - 这是酸的简单测试!
6. Usually react with metals to form salts.
通常与金属反应形成盐。
7. Acids contain hydrogen ions.
酸中含有氢离子。
8. Turn Universal Indicator from green to red, and have a pH less than 7.
将通用指示剂从绿色变为红色,并且 pH 值小于 7。
Examples of acids: are vinegar (ethanoic acid) and lemon juice (citric acid)
magnesium + hydrochloric acid -> magnesium chloride + hydrogen gas
镁 + 盐酸 -> 氯化镁 + 氢气
Some common acids used in your laboratories at school will be:
1. Hydrochloric acid, HCl(aq)盐酸,HCl (水溶液)
2. Nitric acid, HNO3(aq)硝酸,HNO 3(aq)
3. Sulphuric acid, H2SO4(aq)硫酸,H 2 SO 4(aq)
Properties of alkalis碱的性质
1. They feel soapy to touch.
他们摸起来有肥皂味。
2. They are soluble bases.
它们是可溶性碱。
3. Like acids, they can burn the skin.
像酸一样,它们会灼伤皮肤。
4. They turn red litmus blue - this is how you test for an alkali!
它们把红色石蕊试金石变成蓝色——这就是你测试碱的方法!
5. Alkalis contain hydroxide ions (OH-).
碱中含有氢氧根离子(OH -)。
6. They taste bitter.
它们尝起来很苦。
7. Turns Universal Indicator from green to blue or purple.
将通用指示灯从绿色变为蓝色或紫色。
Some common alkalis used in your laboratories at school will be:学校实验室中使用的一些常见碱是:
1. Sodium hydroxide, NaOH(aq)
氢氧化钠,NaOH (水溶液)
2. Ammonia, NH3NH4OH(aq)
氨,NH 3 NH 4 OH (aq)
3. Calcium hydroxide, Ca(OH)2(aq)
氢氧化钙,Ca(OH) 2(aq)
Properties of neutral substances中性物质的性质
1. Litmus paper is not affected by neutral paper.
石蕊试纸不受中性纸影响。
2. Tend to be harmless
趋向于无害
3. Universal Indicator stays green.
通用指示灯保持绿色。
Common examples of neutral substances:中性物质的常见例子:
1. Water水
2. Sodium chloride solution, NaCl(aq)(common salt)氯化钠溶液,NaCl (aq)(食盐)
3. Sugar solution C6H12O6(aq)糖溶液C 6 H 12 O 6(aq)
The pH scale
The Strength of an Acid酸的强度
Acids and alkalis can be strong or weak!酸和碱可强可弱!
So how can we measure their strength?那么我们如何衡量他们的实力呢?
The strength of an acid or alkali is shown using a scale of numbers called the pH scale. The numbers go from 0-14.酸或碱的强度使用称为pH 标度的数字标度显示。数字从0 到14。
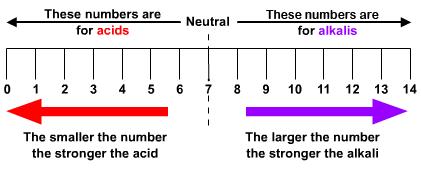
On the scale it follows that:在规模上是这样的:
An acidic solution has a pH number less than 7
酸性溶液的 pH 值小于 7
An alkaline solution has a pH number greater than 7
碱性溶液的 pH 值大于 7
A neutral solution has a pH number of exactly 7.
中性溶液的 pH 值正好为 7。
You can find the pH of any solution using universal indicator. Universal indicator is a mixture of dyes. It comes as a solution or in paper.
您可以使用通用指示剂找到任何溶液的 pH 值。通用指示剂是染料的混合物。它以解决方案或纸质形式出现。
Universal indicator will change from green to a different colour depending on the pH of the solution you place it in.
通用指示剂会根据您放置的溶液的 pH 值从绿色变为不同的颜色。
Note:
In a strong acid, nearly all the acid molecules form ions.
在强酸中,几乎所有的酸分子都会形成离子。
In a weak acid, only some of the acid molecules form ions.
在弱酸中,只有一些酸分子形成离子。
The more OH- ions (hydroxide ions), the more alkaline an alkali will be.
OH -离子(氢氧化物离子)越多,碱的碱性越强。
In other words, the more OH- ions there are the higher the pH number.
换句话说,OH-离子越多,pH值就越高。
版权声明:CosMeDna所有作品(图文、音视频)均由用户自行上传分享,仅供网友学习交流。若您的权利被侵害,请联系删除!
本文链接://www.cosmedna.com/article/916594169.html